How the Immune System Works (And Why Herbalists Need to Know)
understanding immunity, autoimmunity and the path towards balance
When most people think of the immune system, they picture something vague — a defense wall, maybe, or a battalion of white blood cells marching into battle.
In the herbal world, it’s often reduced to a buzzword: boost immunity with this tincture, that tea, a few capsules of dried roots and bark. But the immune system is far more intricate, far more relational, and far more powerful, for better or worse.
It is a system of discernment. Of balance. Of knowing who you are and who you are not.
And if we want to work with herbs in a way that’s wise, responsible, and truly supportive, especially when it comes to immunity, we need to understand the terrain we’re working within.
What is the immune system actually doing when we take elderberry during a cold? Or astragalus for long-term support? What happens when the immune response is under-functioning… or overzealous?
This article is a primer. Not just on how the immune system works, but what herbalists should know to support it more intentionally.
Whether you're just beginning your journey with plant medicine or guiding others through their own, consider this your foundation: the inner workings of the body’s first responders, memory keepers, and quiet regulators.

The Immune System at a Glance: Harmony, Not War
When we hear the words “immune system,” most of us picture an army: white blood cells charging in, barriers holding the line, invaders being neutralized and destroyed. And while there’s truth to that imagery, it’s also a little outdated.
Because the immune system isn’t just about war. It’s about discernment. Communication. Knowing when to act, when to rest, and when to leave things alone.
At its core, the immune system is a wide-reaching network of tissues, cells, and chemical signals designed to protect the body without harming it in the process. And rather than one single defense line, it actually operates through three interlocking layers:
Anatomical and physiological barriers – skin, stomach acid, mucus membranes, tears, and the microbiome
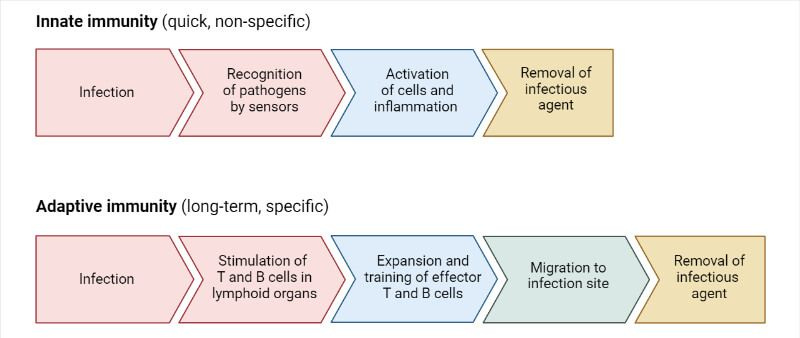
Innate immunity – the fast, non-specific system that acts immediately
Adaptive immunity – a slower, smarter response that learns and remembers
Together, these systems help us filter out what doesn’t belong — whether it’s a virus, a toxin, or a damaged cell — and maintain balance in the body’s internal environment.
So, who’s actually doing the work?
Let’s meet a few of the main players.
The Cells That Keep You Safe (Even When You Don't Know It)
The innate immune system is like the neighborhood watch. It’s been around since the beginning (literally - even jellyfish have it), and it jumps into action the moment something seems off.
It includes cells like:
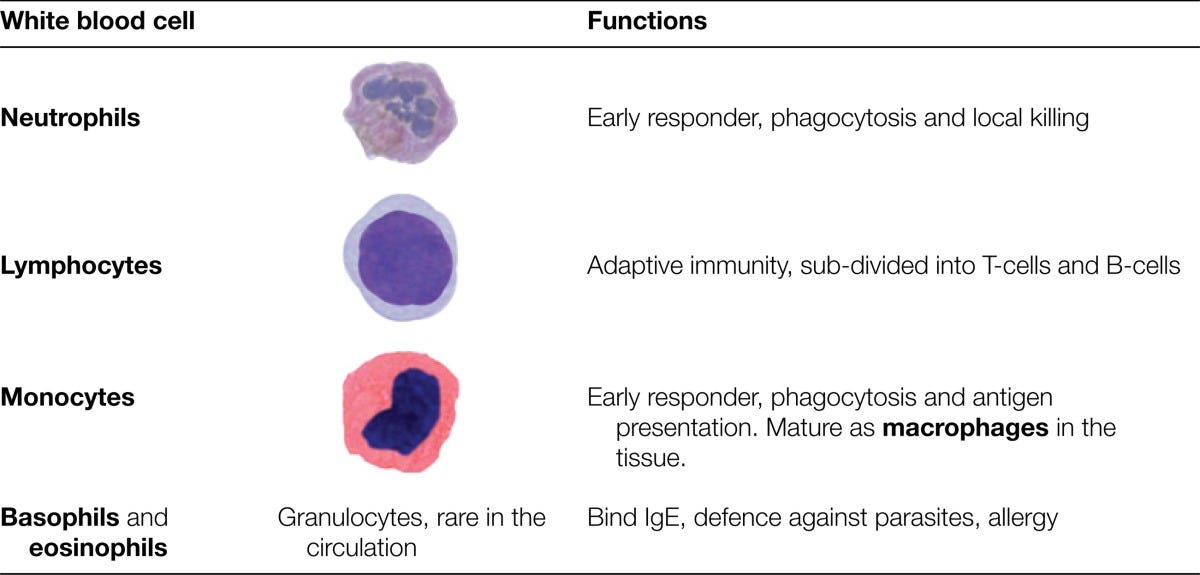
Neutrophils, your first responders. These are the most abundant white blood cells, and they waste no time in engulfing bacteria or damaged tissue.
Macrophages, the cleanup crew. They swallow pathogens and debris and also help sound the alarm for backup.
Dendritic cells, the messengers. They collect information from the scene and deliver it to the adaptive immune system.
Natural killer (NK) cells, and yes, that’s their real name. They specialize in destroying cells that have gone rogue, like cancer cells or virus-infected ones.
Even your epithelial cells (the ones lining your gut, lungs, and skin) have immune-sensing abilities, detecting invaders and releasing chemical signals to call for help.
immune cells
Then there’s the adaptive immune system, which operates more like an elite task force. These cells aren’t born knowing what to fight. They learn. They remember. They specialize.
T cells help coordinate the response or directly kill infected cells.
B cells produce antibodies, which are proteins that mark invaders for destruction and prevent them from causing more harm.
This side of the immune system is powerful, but it takes time. Days, sometimes. That’s why the innate system always responds first, and why the two systems are constantly talking to each other.
Innate Immunity: Your Body’s Neighborhood Watch
The innate immune system is like a really attentive neighbor. The kind who notices a strange car parked outside or a flickering light in the hallway before anyone else does. It may not know exactly who the intruder is, but it knows something doesn’t belong. And it reacts fast.
This system is with you from birth. It’s hardwired into your cells, tissues, and barriers. Always on call. No training required. While it doesn’t have the tailored precision of the adaptive immune system, it doesn’t need it. Innate immunity is about speed, containment, and coordination.
The Skin and Mucosa: More Than Just a Wall
The first part of your innate immune defense isn’t even made of immune cells. It’s your barrier tissues.
Your skin acts as both a physical wall and a chemical shield. The outermost layer is packed with keratinocytes - tightly linked cells that don’t just block pathogens but also recognize them using built-in receptors. These cells can release cytokines (immune messengers) and antimicrobial peptides, which trigger inflammation and help kill microbes before they get far.
Sebaceous glands (think oily skin and hair) release fatty acids that make the surface of your skin acidic. It’s a hostile environment for most microbes.
Mucous membranes, found in the digestive, respiratory, and urinary tracts, form a continuous layer of epithelial cells that secrete defensins. These small peptides poke holes in microbial invaders.
In your lungs and sinuses, cilia - tiny hair-like structures - sweep away trapped dust, microbes, and mucus. It works like a conveyor belt that clears out what doesn’t belong.
Even at this outer layer, innate immunity is active, not passive. Your barrier tissues are constantly sensing and responding.
Skin as immune barrier
If Something Gets Through...
When a microbe slips past the outer barriers, the innate immune cells take over. This is where things escalate quickly.
You’ve already met the main players:
Neutrophils (the first on the scene)
Macrophages (the cleanup crew)
Dendritic cells (the messengers)
NK cells (the enforcers)
But what do they actually do?
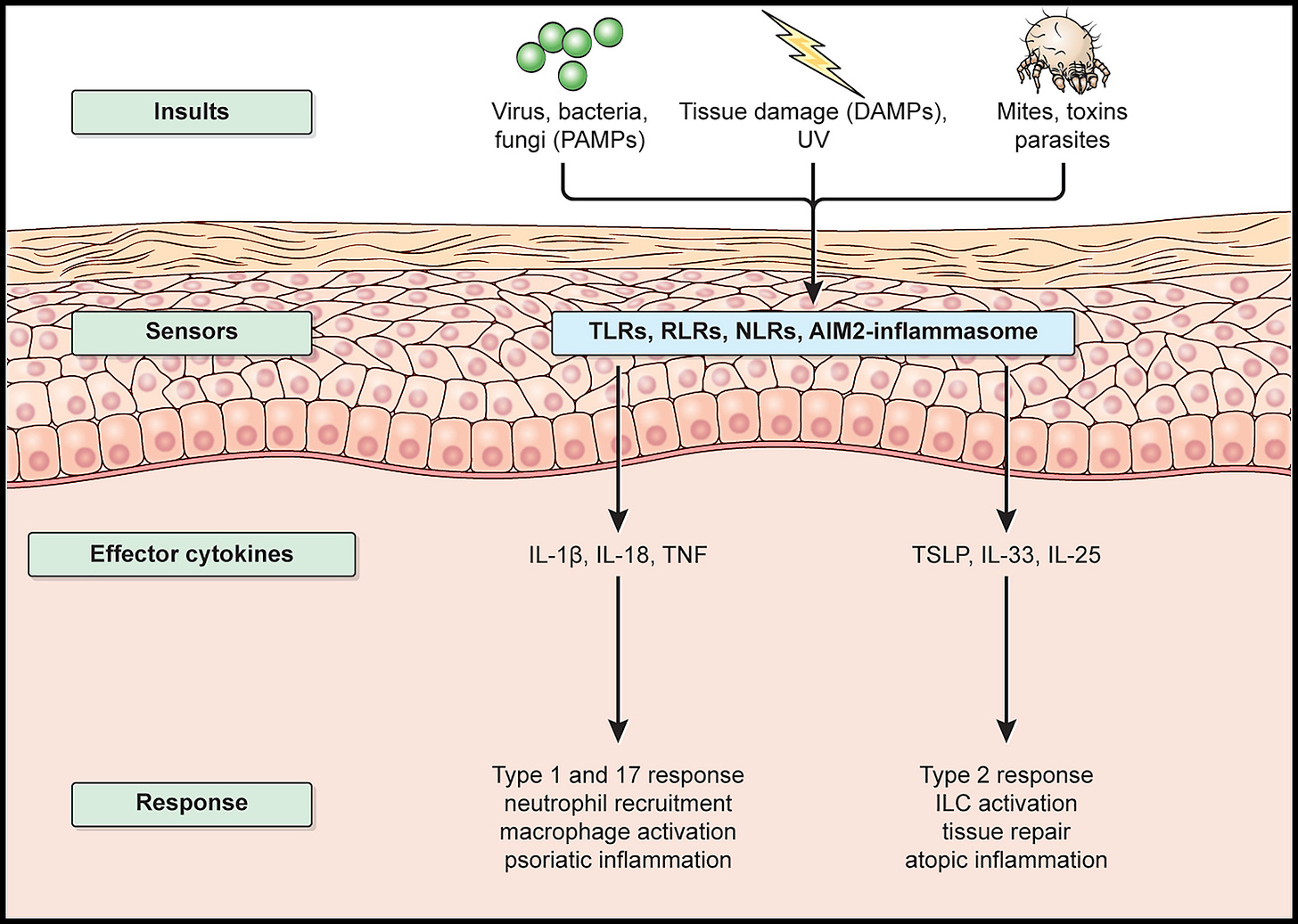
These cells are constantly scanning for signs of danger. Not by recognizing the exact identity of a microbe, but by picking up on shared patterns that pathogens tend to carry. These patterns are called PAMPs (pathogen-associated molecular patterns), and innate immune cells have receptors designed to find them.
There are other signals too. DAMPs (damage-associated molecular patterns) are distress calls sent out by your own injured or stressed cells.
And sometimes, cells are flagged by what’s missing. For instance, NK cells look for healthy surface markers that infected or cancerous cells often stop displaying.
Once activated, these immune cells launch a coordinated response:
They release cytokines and chemokines, chemical signals that call in reinforcements.
They activate the complement system, a protein cascade that tags pathogens for destruction or breaks them open directly.
Blood vessels in the area become more permeable, allowing more immune cells to enter the site of infection or injury.
The result? Inflammation. Redness, swelling, heat, and sometimes pain. It’s not just a symptom - it’s part of the healing strategy.
Built Into Your Tissues
Innate immunity isn’t just floating around in your bloodstream. It’s woven into your tissues.
Your skin, lungs, gut, and even your eyes contain local immune responders. In the gut and lungs, you’ll find specialized immune cells like gamma-delta T cells and B-1 cells. These produce natural antibodies like IgM, which help neutralize microbes quickly—even without previous exposure.
In conditions like atopic dermatitis, where the skin’s barrier function and immune signaling break down, you can see what happens when innate immunity falters. Inflammation lingers. The microbiome shifts. External triggers become harder to tolerate. It’s a reminder that these systems don’t work in isolation. They rely on one another to maintain balance.
No Memory, No Delay
Unlike the adaptive immune system, which takes time to build a personalized response, innate immunity reacts immediately. It responds within minutes or hours. It doesn’t remember pathogens, and it doesn’t adapt to new ones.
But it doesn’t have to. Its job is to hold the line. To respond broadly and quickly. To sound the alarm when something is off. And to create the conditions for the next phase of the immune system to step in if needed.
That next phase is adaptive immunity. It’s where precision and memory come into play.
We’ll explore that next.
Adaptive Immunity: The Memory Keepers
If the innate immune system is your neighborhood watch - always scanning for trouble and acting fast - adaptive immunity is more like a custom-built security system. It’s slower to respond, but once activated, it builds a detailed file on every intruder and remembers exactly how to handle them next time.
Unlike the broad, pattern-based responses of innate immunity, adaptive immunity is precise. It learns. It remembers. It builds targeted attacks tailored to each specific pathogen. But that kind of precision takes time.
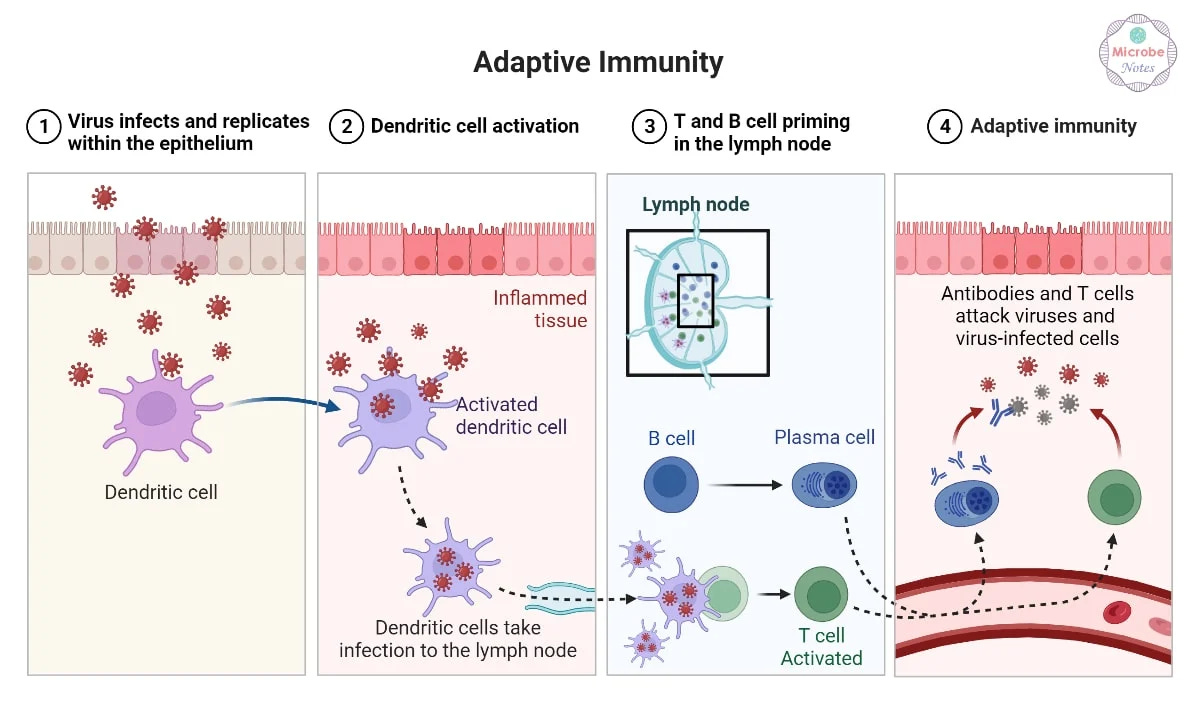
This branch of the immune system is made up of T cells and B cells - highly specialized lymphocytes that recognize unique features of invading microbes. Each one carries its own antigen receptor, designed to match just one type of threat.
Training for Specificity
B and T cells are formed in the bone marrow, but they mature along different paths. T cells travel to the thymus, where they’re trained to distinguish between self and non-self. B cells stay in the bone marrow and develop the tools to create antibodies.
Their receptors aren’t inherited the way innate immune receptors are. Instead, they’re formed through a genetic shuffling process — rearranging gene segments to create millions of possible antigen receptors. This incredible diversity means that, in theory, your body can recognize almost any pathogen it ever encounters.
Of course, randomness comes with risk. Some of these receptors might accidentally recognize your own tissues. That’s why the body has built-in safety checks to remove or silence self-reactive cells. When those checks fail, autoimmune conditions can develop.
The T Cell Response: Recognition and Regulation
T cells don’t recognize floating antigens. They rely on antigen-presenting cells -like dendritic cells or macrophages - to digest a microbe and present its fragments on the cell surface using proteins called MHC molecules. There are two types of MHC:
Class I MHC presents antigens to cytotoxic T cells, which destroy infected or abnormal cells.
Class II MHC presents antigens to helper T cells, which coordinate the broader immune response.
Once a T cell receptor recognizes its matching antigen-MHC complex, it activates. Some become cytotoxic T cells that kill infected or cancerous cells. Others become helper T cells, which release cytokines that guide the immune response - helping activate macrophages, stimulate B cells, or promote inflammation.
A third group, regulatory T cells, helps keep the whole system balanced by suppressing unnecessary or harmful immune responses.
B Cells and Antibodies: The Secret Weapon
B cells recognize antigens directly. Once activated, they multiply and differentiate into plasma cells, which produce antibodies, and memory B cells, which stick around in case the same pathogen returns.
Antibodies are proteins that bind to specific antigens. They help neutralize pathogens in several ways:
Blocking viruses and toxins from entering cells
Coating microbes so phagocytes can recognize and destroy them
Activating the complement system to break down pathogens
There are five major types of antibodies, each with its own specialty:
IgM is the first responder, quick but short-lived
IgG provides long-lasting protection and is the most abundant in blood
IgA guards mucous membranes
IgE plays a role in allergic responses and fights parasites
IgD is less well understood, but thought to help regulate B cell activity
step by step adaptive immune response
The Gift of Memory
After an infection is cleared, most activated T and B cells die off. But a few stick around as memory cells, ready to launch a faster and stronger response if the same invader returns. That’s why adaptive immunity gets stronger over time. It learns from each encounter.
This is the principle behind vaccines: exposing the immune system to a safe version of a pathogen so it can prepare in advance — no illness required.
Adaptive immunity may be slower to start, but it’s deeply efficient. It turns a single encounter into lifelong knowledge. And it ensures the next time your body sees that virus, bacteria, or toxin, it won’t have to start from scratch.
Next, we’ll explore how the immune system decides what belongs and what doesn’t — and what happens when that recognition fails. Because knowing how to fight is important, but knowing who to fight? That’s everything.
To compare:
immunity comparison chart
When the Immune System Loses Its Compass
The immune system is powerful. It can dissolve bacteria, dismantle viruses, neutralize toxins, and even eliminate cancer cells. But with all that strength, there’s one very important question it has to answer before taking action:
Is this a threat, or is this me?
This ability to tell the difference between “self” and “non-self” is called self-tolerance, and it’s one of the most essential functions in the immune system. When it’s working, your immune system keeps you safe without harming your own tissues. When it fails, things can get messy.
The Breakdown of Tolerance
Autoimmune conditions like lupus, Type 1 diabetes, Hashimoto’s thyroiditis, and rheumatoid arthritis all share one thing in common: the immune system starts mistaking the body’s own cells for invaders. What was once a finely tuned defense system becomes confused, misdirected, and hard to rein in.
These conditions aren’t temporary.
Many autoimmune diseases show up early in life and tend to stick around, often requiring long-term care and ongoing management.
And they don’t just affect the body - they impact energy, digestion, hormones, mental clarity, and quality of life, often during the prime of someone’s working or reproductive years.
Modern medicine has brought some powerful tools to the table. Medications that block specific immune messengers, like TNF-alpha inhibitors, have changed the course of diseases like rheumatoid arthritis and inflammatory bowel disease. These treatments help lower inflammation and ease symptoms.
But many of these medications only work on the later stages of disease, when inflammation has already taken hold. They don’t necessarily address the original imbalance that started it all. And because of this, people often stay on these drugs for years or even decades, sometimes facing side effects like increased infections or a suppressed ability to fight off future threats.
Three Phases of Autoimmunity
Researchers now describe autoimmune disease as unfolding in three phases:
1. Initiation
This is the spark, where a combination of genetic susceptibility and environmental triggers (like viral infections, toxins, or gut barrier issues) causes the immune system to become dysregulated. People often don’t have symptoms during this phase, even though the immune confusion has already started.
2. Propagation
This is where symptoms begin. Inflammatory messengers are released, tissue is damaged, and immune cells continue to respond even after the original trigger is gone. The system has lost its brakes. You may hear terms like epitope spreading or effector/regulatory imbalance, which describe how the immune reaction grows and becomes harder to stop.
3. Resolution
Ideally, the body begins to restore balance, using internal systems like regulatory T cells to quiet the inflammation and guide healing. But for many people, this balance is only partial or short-lived. There’s still a constant push and pull between inflammation and regulation, which leads to flare-ups or relapses.
Each phase reflects a breakdown in the immune system’s ability to distinguish friend from foe. And without a strong resolution phase, the inflammation becomes chronic and self-sustaining.
Why Herbalists Should Care About Self vs. Non-Self
When we talk about supporting the immune system, we often default to the idea of “boosting.” But in the context of autoimmunity, more stimulation isn’t always better. Sometimes the immune system is already doing too much, just targeting the wrong thing.
This is where herbal strategy becomes nuanced.
Plants that modulate the immune system, rather than simply stimulating or suppressing it, can offer real support.
Think of herbs like Reishi, Turmeric, Licorice, and Schisandra. These herbs can encourage immune balance, calm overreactions, and support inflammation resolution.
Others, like Elderberry or Echinacea, may be better suited to short-term support during infection, not for long-term use in someone with active autoimmune disease.
Understanding where someone is in the autoimmune process, whether they’re flaring, recovering, or trying to prevent another spiral, helps us choose herbs that meet the moment.
It’s not about forcing the body to fight harder. It’s about helping the immune system remember what safety feels like and guiding it gently back to center.

The Wisdom of the Immune System
The immune system is more than a defense mechanism- it is a living map of the body’s boundaries, memories, and ability to return to center.
It doesn’t just fight. It listens. It learns. It holds a history of everything you've ever encountered, and every time it chooses to act - or not - it's making a decision about what belongs.
For herbalists, this isn’t just academic.
It’s a reminder that every plant we work with is entering a conversation already in progress. And the more we understand the rhythms of immunity - its patterns of urgency, quiet, confusion, and repair - the better we can offer support that’s not just reactive, but wise.
There’s beauty in that kind of knowing. And there’s responsibility too.
If you found this helpful, make sure to like this post to show support. It helps more folks find this work.
You can also share this post with a friend or colleague who might enjoy it, or leave a comment below to join the conversation. I always love hearing your thoughts.
In the next piece, we’ll step into the realm of immune modulation — where herbs meet nuance, and where healing often lies not in doing more, but in doing less with deeper intention.
This upcoming guide is created especially for members of The Buffalo Herbalist Community and will include a companion printable and extended lesson exploring herbal strategies for immune regulation, resilience, and the deeper layers of immune function we didn’t have space to cover here.
If that sounds interesting for you and you’d like to get in on this information - you can upgrade your subscription here:
I totally understand if another monthly subscription isn’t in the cards for you right now. If you’d like to support this publication in another way, you can ‘buy me a coffee’ here:
Thank you for reading, and for being part of this space.
Until next time,
Agy | The Buffalo Herbalist
Bibliography
Marshall, J. S., Warrington, R., Watson, W., & Kim, H. L. (2018). An introduction to immunology and immunopathology. Allergy Asthma and Clinical Immunology, 14(S2). https://doi.org/10.1186/s13223-018-0278-1
Chaplin, D. D. (2010). Overview of the immune response. Journal of Allergy and Clinical Immunology, 125(2), S3–S23. https://doi.org/10.1016/j.jaci.2009.12.980
Nicholson, L. B. (2016). The immune system. Essays in Biochemistry, 60(3), 275–301. https://doi.org/10.1042/ebc20160017
Turvey, S. E., & Broide, D. H. (2009). Innate immunity. Journal of Allergy and Clinical Immunology, 125(2), S24–S32. https://doi.org/10.1016/j.jaci.2009.07.016
Grubbs, H., & Kahwaji, C. I. (2023, August 14). Physiology, active immunity. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK513280/
Aristizábal, B., & González, Á. (2013, July 18). Innate immune system. Autoimmunity - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK459455/
Catania, L. J. (2022). The adaptive (aka “acquired”) immune system. In Elsevier eBooks (pp. 25–43). https://doi.org/10.1016/b978-0-323-95187-6.00006-6
Rosenblum, M. D., Remedios, K. A., & Abbas, A. K. (2015). Mechanisms of human autoimmunity. Journal of Clinical Investigation, 125(6), 2228–2233. https://doi.org/10.1172/jci78088




Thanks! Perfect timing and baby and I just got over something!
I really appreciate your teaching skills. You take very complex systems and help my brain to understand. Thank you!